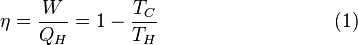Its time for a sanity check. If this check-valve device were possible, you could install it on the intake manifold of a piston engine, then proceed to extract massive amounts of mechanical power. The machine would ingest atmospheric air, output usable mechanical work, and exhaust nothing but cold air.
In the engineering profession, we call this idea a Clausius Violator of the Second Kind in that its impossible to construct a device that extracts work from a single thermal reservoir (see the Second Law of thermodynamics). Not only would the device demand that heat flow from cold-to-hot, it would also require system entropy to decrease - both of which are not possible.
I took gas dynamics in college. It was one of my favorite classes. I really liked the topic of supersonic nozzle design, and also determining shock-angles from various object geometries within super-sonic flight. FWIW, if an engineering student proposed a free-energy check-valve device within a gas-dynamics class - the Dean of Engineering would probably kick the student out of the engineering program for life. Does this seem extreme? Ask yourself the following question: would you want the engineer who designed the wing of the airplane youre riding in, to (A) believe in imaginary machinery and impossible technology? Or (B), have a firm understanding of the laws of physics, and capable of differentiating between achievable and impossible machine designs?
Heat flows from hot to cold. Entropy always increases. Absolute zero is absolute. There is no such thing as free energy - as perpetual motion is not possible.
I will make one more post on this topic, Entropy455, and then I will simply stop.
1st point.
The entire post above summarizes succinctly our knowledge about thermodynamics acquired in the last two centuries. My argument about "Boltzmann's demon" is a *thought experiment* (are you familiar with those ?) used by Peter Atkins in his famous book "The Second Law" to lead to the conclusion that all you write above is exactly true. We are disagreeing on exactly nothing in this discussion, except on your calling negative temperatures "hogwash". You know, thought experiment in the sense of "if you could construct a one-way valve - but you can't, because if you could, x, y and z would follow, and we know that that is impossible". This book by Peter Atkins (which I heartily recommend) also derives the existence of negative temperatures if I remember correctly, and this was in 1984. It follows from the mathematics employed, you can do the calculations yourself on the back of an envelope, but instead of opening your eyes and seeing, you rather choose to parrot what you have heard in a thermodynamics class I don't know how many years ago. Use your imagination, for heaven's sake!
2nd point.
Please understand there is a difference between the interpretation of a scientific article by journalists and what the authors actually wrote. The sentence you quoted in your other post right after the one above is nowhere to be found in that article. I'll PM you a copy if you like to see for yourself. If it would have been in there, perhaps because all the authors would have been delusional nutcases, the peer review process to which all scientific articles are subjected prior to publication would have very quickly weeded it out. And incidentally, the authors do a pretty good job at explaining the basics behind it too, so perhaps you should at least read the introduction of the article for yourself.
Whatever interpretation journalists give to it is mainly meant to be thought-provoking - or perhaps provocative, especially to people like yourself I suppose. They do not mean at any time that this could be a practical way to a free energy machine. Because those don't and can't exist. Yes, we know you know that. And I know it too. And so do these authors. The journalist who wrote that sentence - well perhaps. I do hope so.
3rd point.
I especially take offense at the mention of me being kicked out of an engineering curriculum for life by the Dean on mentioning "Boltzmann's demon" - which, once more, is a very instructive thought experiment to demonstrate the validity of the second law of thermodynamics - it says you cannot win, even if you try to cheat. You really should read that Atkins book, and get rid of this "It cannot be done because it's the (second) Law" attitude, and instead try to breed a little understanding of the reasoning, process and mathematics behind it.
And that is where my contribution to this shouting match will end. I think I'll go do some model engineering to "cool down" (hee hee).